
Humboldt Penguin |
Distribution / General: |
||||||
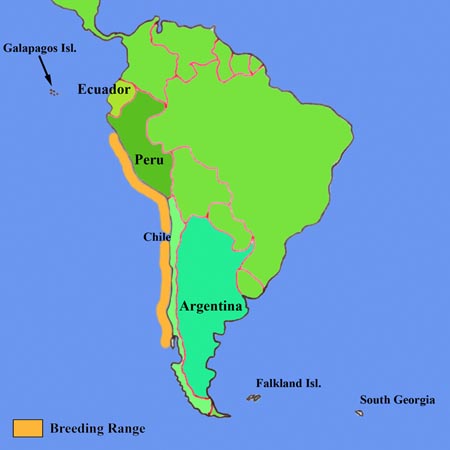
The Humboldt Penguin (also sometimes referred to as the Peruvian Penguin), along with the Galapagos, Magellanic and African Penguins, belongs to the Spheniscus genus of penguins. It is endemic to the regions along the west coast of the South American continent where the nutrient-rich cold Humboldt / Peruvian current flows northwards along the coast. Its breeding range currently extends over 4500 km of coastline, from as far north as La Foca Islands (5'12 S) in northern Peru to the Punihuil Islands (42'73 S) off Chiloe in Southern Chile, where it overlaps with that of the Magellanic Penguin and small mixed breeding colonies can be observed. The northern populations are miniscule and the first significant colony at the northern end of the range is at Pachacamac Island (12' S) where several hundred birds have been counted in recent years. Further significant colonies along the Peruvian coast are at Tres Puertas, San Juanito Island, Punta San Juan and the Hornillos Islands, with the mainland colony at Punta San Juan harbouring about 36% of the total population for Peru of 4425 estimated in 1999-2000 (Paredes et al., 2003. Waterbirds 26(2), p.129-256). In Chile, the largest population is found on Isla Chanaral (29'02 S), where recent data suggests that numbers may be as high 29000 adult birds, although only about 16000 were present on the island at the actual time of the count (Mattern et al., 2004. Waterbirds 27(3), p.368-376). This by far surpasses previous estimates and raises questions about the accuracy of population censa of this penguin in general.Populations are subject to significant fluctuations as the result of the huge declines observed following El Nino Southern Oscillation (ENSO) events. These are associated with higher sea temperatures and absence of prey species, resulting in mass starvation of Humboldt Penguins. For example, the 1982/83 ENSO event is thought to have reduced the total adult population size from levels of around 20000 down to less than 6000 (Birdlife International 2003. Web Resource: Rep. on Status and Conserv. of Humboldt Peng.).
|
 |
 |
  |
  |
|
Humboldt Penguins, Munich Hellabrunn Zoo |
Humboldt Penguins, Munich Hellabrunn Zoo |
Feeding: |
||||||||||||||||||||||||||||||||||||||
Humboldt Penguins generally feed at shallow depths on schools of anchovies, sardines, araucanian herrings or silverside. This puts them in direct competition with commercial fisheries. As in other penguin species, the proportions of prey species taken at a given location and time may vary substantially depending on temporal food availability and other prey types may be taken in large numbers under certain conditions.DietA detailed study was performed on the diet of Humboldt Penguins at Pan de Azucar Island in 1997/98 and the Punihuil Islands during the 97/98 and 98/99 breeding seasons (Herling et al. 2005. Mar. Biol. 147, p.13-25). Stomach contents were flushed from birds returning to colonies in order to analyse their composition. At Punihuil, the penguins primarily consumed anchovies (Engraulis ringens) in 97/98, yet fed mainly on silverside (Odontesthes regia) in the subsequent season. In both seasons araucanian herring (Strangomera bentincki) was the second most common prey item. In contrast, at Pan de Azucar garfish (Scomberesox saurus) was the main prey item. Garfish was also previously found to be the predominant prey item at Isla Chanaral slightly further south (Wilson et al. 1995. J. Ornithol. 130, p.75-79). Stomach samples at both Pan de Azucar and Punihuil contained crustaceans (isopods and stomatopods) and cephalopods such as the squid Loligo gahi, although these were not significant in terms of mass except for in one sampling period at Pan de Azucar, where cephalopods made up 36% of the diet. This may have reflected a shortage of fish in the wake of the 1997/98 ENSO event. Large numbers of stomatopods were also consumed at this time (31% of prey items), although due to their small size they only contributed minimally in terms of mass.Table.1 Composition of diet by wet mass (%) Extracted from Tables 3, 4 (Herling et al.):
The majority of fish obtained from stomach samples were in the range of 10-20 cm long. Anchovies and herring had a mean length of about 10 cm and weighed about 8-12 g at Punihuil, although they were only about 8 cm long and under 4 g on average at Pan de Azucar. Silverside were generally about 20 cm long and Garfish 15-20 cm, weighing about 50 g and from 6-15 g on average, respectively. The largest prey items caught were common hake (Merluccus gayi) with a length of around 30 cm and a mass over over 150 g. This shows that Humboldt penguins are able to consume prey items ranging from tiny crustaceans up to quite large fish. |
 |
 |
 |
|
Lingual papillae in mouth, Munich Zoo |
Lingual papillae in mouth, Munich Zoo |
Lingual papillae on tongue, Munich Zoo |
 |
 |
|
Swimming Humboldt Penguin, Munich Zoo |
Diving Humboldt Penguin, Munich Zoo |
Reproduction: |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Nest & Partner SelectionThe preferred nesting sites of the Humboldt Penguin, at least in northern part of its range, are cliff-top sites with thick layers of guano, into which the penguins excavate nesting burrows to protect themselves and their brood from the sun and predators. Nests are often lined with small amounts of feathers or seaweed which are collected by either member of the pair. Guano essentially consists of the accumulated excrements of the so-called guano-birds, which are the Guanay Cormorant and the Peruvian Pelicans and Boobies. In the past, many cliff-tops and small islands were covered in many meter thick layers of guano. However, following intense guano harvesting by man in the last centuries, much of this has been removed. As a result, many nests are now found on the surface, on beaches or in caves. The nesting preferences of Humboldt Penguins at Pta. San Juan, where guano harvesting has been partially reduced in recent years, was studied in detail between 1993 and 1996 (Paredes and Zavalaga 2001. Biol. Conserv. 100, p.199-205). At Pta. San Juan, the vast majority of nests were located on cliff-tops (85%), and of these more than 90% were surface nests. Some burrow nests were also observed. The remaining nest sites were distributed on slopes, where the number of nests in natural crevices or in burrows was somewhat higher. There was a strong correlation between average guano depth and the number of burrow nests. The number of burrow nests increased over the study period as the layer of guano gradually thickened following a number of years without a harvest. Cliff-top burrow nests produced highest numbers of fledglings / nest (1.4 annually), whereas on the sloping sites the crevice nests (which nest-type is not found on the cliff-top) appeared marginally better than the other nest types, but all ranged between about 0.95 and 1.15 fledglings / nest. Beach nests were on average the least productive, mainly as the result of sporadic flooding incidents due to high ocean swells, although in flooding-free years productivity can match that at cliff-top sites. Cave nests, which accounted for only 6% of the breeding sites at Pta. San Juan, were not assessed. At many often-disturbed sites, cave nests account for the majority of nesting sites.
In one study, the temperature of birds in burrow nests was compared to those in nests amongst rocks. The nests in burrows were found to be warmer. Chicks born into warmer nests were able to maintain their own body temperatures earlier and suffered lower mortality (Soto-Gamboa et al. 1999. Rev. Chilena de Hist. Nat. 72(3), p.447-455).Penguins affected by ocean swells tended to choose other nesting sites in subsequent years and may thus have largely been inexperienced breeders. Once a successful nesting site has been found, Humboldt Penguins are generally considered quite faithful to it. In one detailed study, it was found that about 60% of breeding adults reoccupy nests used in the previous breeding season, whilst about 30% moved to a nearby site and 10% nested at a different part of the colony (Teare et al. 1998. Penguin Conserv. 11, p.22-23). Migration of birds between colonies appears to be so low that it can hardly be detected by direct monitoring of marked birds, yet studies of the genetic diversity of the penguins at the different sites shows that there is sufficient gene flow between populations to keep the level of genetic differentiation between different sites at a low level (Schlosser et al. 2009. Conserv. Genet. 10, p.839-849).Humboldt penguins can fiercly fight for nesting sites. Usually this only leads to small lacerations on their faces, however in rare cases fatal injuries can be inflicted. The images below show the result of a fight for a nesting cave at Munich Hellabrunn Zoo. The injured penguin was losing significant amounts of blood with no sign of the bleeding abating after 30 mins. The notified zoo veterinary staff retrieved the penguin from the enclosure to treat it. Fortunately the penguin survived, yet in the wild it is almost certain that the penguin would have bled to death.
Humboldt Penguins are considered relatively faithful to their partners although they will readily establish new pair bonds if the partner is lost. Certain forms of behaviour are associated with courtship and pair-bonding. Courtship and copulation in general has been documented in detail thanks to a study on extra-pair copulations at the Pta. San Juan colony (Schwartz et al. 1999. Behav. Ecol. 10(3), p.242-250). Courtship commences up to 10 days before copulation, with a mean of 1.77 days. The main courtship display is the bray call which is generally performed as the penguin points its open beak skywards whilst gently flapping its flippers (This is essentially like the Ecstatic Display observed in Pygoscelis Penguins). The bray is performed by both sexes in the Humboldt Penguin, although probably slightly more commonly by males advertizing their territories.
Once a pair bond has been established, this display may be performed mutually. Mutual preening (allopreening), bill-duelling (both birds face each other and vigorously shake their heads resulting in clattering of their bills against each other) and flipper patting by the male bird are further manifestations of pair bonding.
Mutual displaying and bill-duelling often occur when one partner returns to the nest. Bill-duelling was always followed by flipper-patting. Flipper-patting is largely performed with the male aside of or behind the female and in 21% of cases evolved into a copulation attempt. The male uses his bill to push the neck of the female downwards, gently forcing her to the ground before finally mounting. The male stands on the back of the female, steadying itself using its flippers. Cloacal contact is established when the female lifts its tail feathers and the male edges backwards, arching its rear slightly and dipping its cloaca onto that of the female. Most copulations lasted for more than 2 minutes unless aborted, which occurs in about 25% of cases. Copulation was not observed between 9:00 and 16:00, probably mainly as the birds were preoccupied with foraging. Nearly 90% of within-pair copulations occurred at the home burrow. All copulations (including extra-pair ones) were observed prior to egg-laying. Indeed, on average the last copulations occurred about 10 days before laying of the first egg. This suggests that penguins, as many other seabirds, are able to store sperm for significant lengths of time.
Regarding extra-pair copulations, the study found that 19% of males and 31% of females engaged in these, yet genetic analysis of the offspring revealed no evidence of extra-pair fertilizations. Males generally performed these at their nests, whilst females tended to perform these away from their nests, usually also avoiding directly neighbouring burrows. No particular choice of location was evident. Whether, extra-pair copulation is merely coincidental or serves a particular purpose, such as assessing potential future mates, could not be determined.At Pta San Juan, it has been observed that about 60% of females retain mates from one year to another (Paredes et al. 2002. Auk 119(1), p.244-250). Reasons for mate changes may be loss of the male or divorce. When Humboldt Penguins change mate between breeding seasons, this has no effect on the subsequent date of onset of breeding, whilst mate changes during the breeding season following a successful breeding attempt resulted in laying of a second clutch being delayed by more than a month.Interestingly, nest intrusion behaviour by unmated males has been observed at Pta. San Juan (Taylor et al. 2001. Condor 103(1), p.162-165). The intruding penguins entered nests which were occupied by breeding pairs and displayed aggression against the resident adult and its brood. The behaviour resulted in several incidents of egg loss or chick mortality and was responsible for over 10% of breeding failure at the colony. In two of the five intrusion events observed, the intruding male eventually paired with the resident female. Such behaviour is counterproductive for population recovery as a whole, especially if there is a surplus of male birds which may be the case after ENSO events.
Timing of BreedingThe Humboldt Penguin is relatively unusual in that it has an extended breeding period during which, given suitable conditions, it may rear two successive sets of chicks. This behaviour, which can also be observed in Galapagos Penguins and African Penguins, is probably an adaptation to the climatic conditions encountered in the northern part of its breeding range where marine productivity is extremely high allowing for extended breeding during good years. This can rapidly compensate for the drastic population declines observed following the periodically occurring ENSO events.The timing of breeding is not uniform throughout the breeding range. In particular one can distinguish between Peruvian and northern Chilean populations and those in central Chile.At Pta San Juan, Peru, in 1993-1997, first eggs were laid in mid-march, with the majority (62%) of 1st clutches being laid in April (Paredes et al. 2002. Auk 119(1), p.244-250). A second peak in egg-laying was less distinct but occurred in August or September. This peak is less prominent since whilst birds that laid in April and successfully raised chicks to fledging would often instigate a second breeding attempt at this time, first clutches continue to be laid in small numbers throughout the breeding season and second clutches may be laid earlier due to failure of the first breeding attempt, meaning that breeding becomes less synchronous further into the season. Indeed, some time after large numbers of nests were flooded during two of the study periods, a small increase in breeding could be detected presumably as a result of these birds laying replacement clutches. Further, only 47% of birds lay more than one clutch, thus further contributing to the lesser prominence of the peak. These include 4% of birds that lay 3 clutches which is only possible if at least one of the first two clutches fails early. Some birds are prolific breeders, with 8 out of a group of 11 females studied over 3 years having 5-6 clutches during this period. The mean productivity of the 11 females was 4.54 fledged chicks, i.e. about 1.5 per annum.Focusing on pairs that laid 2 clutches in a year, one found that 73% were double brooders, whilst 27% had failed to successfully raise chicks from the 1st clutch. The double brooders had relatively synchronized second laying periods around the same time every year. The breeding success of the double brooders was 2.61 fledglings / year, with at least one chick being raised by 58% of these pairs during the second breeding attempt. Success rates were highest for birds that had started their first breeding attempt earlier than average. Success rates of single brood pairs were not related to laying date of the clutch.In Chile, the Humboldt Penguin populations also show a bimodal distribution in egg laying dates, yet both peaks are delayed by a month compared to in Peru. One study monitored breeding success at Pajaro Nino from 1995 to 2000 (Simeone et al. 2002. Mar. Ecol. Prog. Ser. 227, p.43-50). This site is only weakly influenced by the Humboldt current and has high annual rainfall concentrated in autumn and winter. Breeding was focused in the April-June (Autumn) and August-January (Spring) periods. The autumn period was regularly marred by heavy rainfall and nest flooding and fewer birds (about 20% less) attempted to nested. During 1996 and 1997, 86 and 94% of nests, respectively, were flooded and deserted. In 1998, breeding was reduced due to an ENSO event. Hence, only in 1999 did significant numbers of chicks from autumn breeding fledge. The overall success rate for the autumn season was only 0.12 chicks / nest. Adult birds generally deserted the colony for 1-2 months following flooding of the nest sites. The spring breeding season was more successful and yielded a mean of 0.52 chicks / nest. In the 1997 season, the onset of Spring breeding was delayed by several weeks due to late rainfall and continued flooding of nests.Interestingly, the 1998 ENSO event reduced the number of breeding pairs by 85 and 55% during autumn and spring breeding periods, respectively, but the birds that were breeding showed only a slight reduction in breeding success. Further, following the ENSO event, water temperatures dropped to below average levels and resulted in above average numbers of breeding pairs and high breeding success. The fact that the number of breeding pairs was high shortly after an ENSO event means that the birds had dispersed but not suffered from significant mortality in this region. The latter is in contrast to more northerly sites which are much more severely affected by ENSO events.
Laying and IncubationThe two eggs are laid about 3-4 days apart and parents alternate in incubation duties during the approx. 42 day incubation period.Nest ReliefIn captive populations, gentle throb calls are made by the incubating bird when approached by its partner (Thumser and Ficken 1998. Mar. Ornithol. 26, p.41-48). These are generally reciprocated by the returning bird.Brood / Guard PhaseChicks hatch with a thin coat of down (protoptile plumage) which allows efficient heat transfer from the brooding parent. A thicker down (mesoptyl plumage) is developed within 2-3 weeks and is greyish-brown with a white chest. The thicker down and other physiological developments mean that the penguin becomes thermoemancipated at this stage and no longer needs to be brooded. The parents return from foraging trips and make food available to the chicks by regurgitation. Larger chicks will tend to get more food, and when little food is available the smaller chick often does not survive. At Pta. San Juan, foraging trips are generally restricted to the daytime during the whole chick-rearing period, meaning that chicks are generally fed once a day. The guard phase may also serve to prevent thermal stress by exposure to the sun in surface nests and to protect from aggression by other adults.
Creche PhaseSince they spend much time in their burrows, young Humboldt Penguins do not generally form creches. Surface-nesting birds tend to guard their chicks until they are nearly ready to fledge.
Acoustic Parent-Chick RecognitionParent-chick recognition has not been studied in detail in the Humboldt Penguin. However, some attention has been paid to it during the study of penguin vocalizations in general in captive populations (Thumser and Ficken 1998. Mar. Ornithol. 26, p.41-48). The chick makes a sound sometimes referred to as a Peep when it is begging for food. This subjectively appears to be individually distinct. Some adult calls also appear to be individually distinct.FledgingHumboldt Penguin chicks generally fledge after about 10-12 weeks. At this age they have fully developed their juvenile plumage and should have near adult weights. After fledging, the juveniles spend several months at sea foraging. At Pta. San Juan, chicks fledge from mid-July through to the beginning of March (Paredes et al. 2002. Auk 119(1), p.244-250).
Whilst the extent of dispersal of the juveniles during this period has not been studied, most will eventually return to their natal colonies where they will moult into adult plumage after about 1 year. The young adults will eventually start breeding after reaching about 4 years of age. Fledging juveniles were tagged with transponder chips at the Pajaro Nino Island colony between 1994 and 2003 (Simeone et al. 2006. J. Ornithol. 147 Suppl. p.98). Of those that could be found, seven were found at their natal colony, but four were found breeding at colonies within 100 km to the north. This demonstrates that a significant number of young adults disperse into other nearby breeding populations.(Further Info in: Zavalaga and Paredes 1997. Humboldt Penguins in Pta San Juan, Peru. Penguin Conservation 10(1), p.6-8)Post-Breeding MoultAt the end of the breeding season, Humboldt Penguins perform their annual moult. This occurs mainly in January in Peru, or February in central Chile. Juveniles tend to moult slightly before adults. It is possible that this serves to avoid aggression from adults. The visible part of the moult process when birds remain on land usually takes about 2 weeks, although feather development under the skin starts before this period. Moulting birds are often found at the coast rather then the nest during the moult period.Adult penguins lose about 30% of their weight during the annual moult and subsequently spend 2-3 weeks at sea regaining condition before returning to the colony in preparation for breeding. The moult occurs in the summer during a period where its main prey (in Peru), the anchovy is most abundant. This is unusual compared to most other penguins, which tend to time their breeding to coincide with maximum prey abundance. The reasons are unclear but may allow the penguin to rapidly recover condition before the long breeding season and could also be to avoid breeding at the hottest time of year (Paredes et al. 2002. Auk 119(1), p.244-250).Changes in hormonal levels have been correlated to the moult in captive Humboldt Penguins. Levels of plasma thyroxine and triidothyronine were high during the moult period, whilst levels of the testosterone and estradiol hormones which are related to sexual activity were depressed (Otsuka et al. 2004. Gen. Comp. Endocrinol. 135, p.175-185). Thyroxine is thought to positively influence feather development. The exact trigger for these hormonal changes is not known but photoperiod is likely to play a major role. |
General Behaviour: |
||||||||||||||||||||||||||||||||||||
In general, the behaviour of the Humboldt Penguin corresponds to that of the other members of the Spheniscus species, such as the more intensively studied Magellanic or African Penguins. The extreme timidity of the Humboldt Penguin and consequent easy disturbance is discussed in detail in the "Threats" section below. The Humboldt Penguin is not only timid, but also shows lower levels of aggression towards conspecifics or keepers than e.g. related Spheniscid penguins in zoo populations. Aggressive interactions often start when penguins move around and come into close proximity of each other. A Yell Call is often emitted by an approached penguin and serves as a warning. If the warning is ignored, aggression may involve pecking and chasing behaviour. Aggression is often directed at younger penguins, possibly as these are more easy targets, yet also maybe as a result of the lower experience of such birds in learning to avoid aggression. In zoo populations, aggressive interactions increase when the penguins are kept in smaller spaces and a distinct pecking order could be observed determining which penguins could stand in the preferred parts of the enclosure (Thumser and Ficken 1998. Mar. Ornithol. 26, p.41-48).
The most commonly observed behaviour in basically all penguin species is preening. Penguins largely preen themselves, although allopreening is also observed in many penguin species, including all Spheniscid penguins. Allopreening is a social behaviour and probably plays little role in maintaining the plumage. Normal preening serves to clean and arrange the feathers, but most importantly to spread preen oils from the uropygial gland at the base of the tail over the whole plumage. These oils are water-repellent and serve to maintain the water-proofing of the plumage. Penguins spend much time preening and performing other maintenance behaviour. Stretching behaviours and scratching with the feet, which often is directed to the back of the head which can obviously not be reached by the bill, are also often observed intermittently during phases of plumage maintenance. The head may also be bent backwards to it can be rubbed against the back, maybe as a means of transferring preen oil to it. Maintenance behaviour is most intense after penguins return to land and may continue, interrupted by short rest phases, for periods lasting more than an hour.
Further, a lot of time on land is spent resting.
|
Threats: |
Climate / ENSO EventsEl Nino Southern Oscillation (ENSO) events take a high toll on Humboldt Penguins and in extreme years may result in over 75% reductions in local populations. Whilst populations have always been exposed to these events and have been able to recover in between, it seems that recently the number of more extreme ENSO events has increased, possibly as a result of global warming. Further, a number of other factors such as habitat destruction / disturbance and competition / direct fatalities by fishery activities make it difficult for populations to recover quickly to pre-ENSO levels. Hence, the Humboldt Penguin population in Chile is classified as Vulnerable and the Peruvian population is classified as Endangered.Humboldt Penguins feed largely on anchovies and sardines, which are plankton-eating schooling fish usually found in cold waters within 50 m of the surface. During ENSO events, these fish are significantly smaller and concentrate in bodies of cold water near to shore at depths of over 100 m, or migrate southwards to colder waters. Hence, their availability is significantly reduced. The severity of such events on local fish stocks is also reflected in SERNAP fisheries statistics which showed e.g. an 84% drop in anchovies caught in Chile in January 1998 compared to the previous year.Humboldt Penguins often abandon their nest sites during ENSO events, leading to almost complete breeding failure, and emaciated corpses are often washed up on beaches. Some of these have as little as 40% of the normal body weight, lack fat deposits and have little remaining sternal muscle (Hays 1986. Biol. Conserv. 36, p.169-180). Satellite-tracking studies have shown that the penguins may respond to ENSO events by migrating southwards (Culik et al. 2000. J. Exp. Biol. 203, p.2311-2322). During the 1997/98 ENSO event, 5 birds from Pan de Azucar Island (26'09 S) were tracked and migrated by up to 895 km. The higher the sea-surface temperature anomaly (SSTA), the greater distance the birds travelled. Further, the total daily dive duration was positively correlated with SSTA, ranging from 3.1 to 12.5 hours when the water was warmest. The 12.5 hours together with the necessary surface intervals mean that under poor conditions the birds were essentially using all daylight hours for foraging. Dive depths were however not significantly deeper, suggesting that the birds were not trying to reach the deeper cold waters.Climatic change bears the risk of the spread of disease vectors to new areas and hence it is possible that wild populations may eventually be exposed to new diseases.PredationHumboldt Penguins have always had to contend with natural predators. Band-tailed Gulls (Larus belcheri) may take small numbers of unguarded eggs and both eggs and penguins may be at risk from terrestrial predators such as Andean Foxes at mainland colonies. The Pta. San Juan site is protected by a concrete wall but due to deterioration of the wall in places, Fox entry has occurred. For example, in 1992 about 30 penguins were taken by foxes (Zavalaga and Paredes 1997. Penguin Conservation, June 1997, p.6-8).Predation at sea is little-studied but seals and sharks are likely to take some penguins. Further, predators introduced by man including dogs, cats and rats present a hazard to penguins and their offspring. Man is however the worst predator.Habitat DestructionIn the early 19th Century, population levels of the Humboldt Penguin may have been over 1 million. An initial drastic decline in penguin numbers can be correlated to the onset of large-scale commercial guano harvesting at seabird colonies along the Peruvian and Chilean coastline. Guano has a significant commercial value as a fertilizer component. The penguins at many sites had dug burrows in thick layers of guano deposited by many previous generations of seabirds. These afforded protection from predation and the sun. During harvesting, the guano was removed down to the underlying rock and penguins and eggs were often removed if found at the sites. Nest sites in thick layers of guano at cliff-tops are the preferred and most productive nesting sites for Humboldt Penguins and breeding success is much lower if penguins nest on beaches or in coastal caves, partially due to the permanent threat of ocean swell flooding these nest sites (Paredes and Zavalaga 2001. Biol. Conserv. 100, p.199-205).In Peru, the governmental guano extraction agency PROABONOS has tried to protect penguins at many of its guano sites since 1909 and regulates the harvest process. Nevertheless, significant disruption is inevitable. Making matters worse, workers apparently often steal penguins and eggs to supplement their incomes. From 1998-2006, an agreement was in place between the Wildlife Conservation Society and PROABONOS, allowing observers to oversee the harvest and preventing guano extraction directly from the penguin colonies at Pta. San Juan. Similar cooperation continues and will hopefully become a model for other sites.Impact of Fisheries IndustryWhilst guano extraction was the driving force behind the initial rapid decline in populations, increasing fisheries activities have also taken their toll. The stock of Peruvian Anchovy collapsed in the 1970s as a result of increasing fisheries activities. Clearly, this has had an impact on penguin numbers. Further, significant numbers of penguins are killed directly by fisheries. In the Valparaiso Region in Chile, at least 605 Humboldt Penguins were trapped and drowned in gill nets between 1991 and 1996. These were presumably largely from the Cachagua and Pajaro Nino colonies (Simeoni et al. 1999. Marine Ornithol. 27, p.157-161). The gill nets are often cast near the shoreline to catch commercially valuable corvinas. Penguins hunting anchovies and sardines in the area are probably unable to see the nets and thus swim into them. Most of the birds were killed in mass mortality incidents, where more than 100 dead penguins were swept up onto beaches within a few days. These birds are removed from the nets and thrown overboard. Many of these birds show the characteristic signs of net entanglement including abrasions of the feet, flippers and base of the bill. In one case, fishermen reported putting penguins into sacks so they would sink to the sea bottom. Actual levels of mortality presumably far surpassed that reported since not all fishermen are cooperative and many carcasses will not be found. A further study of the bycatch of small scale fisheries at Pta. San Juan also revealed high levels of mortality (Majluf et al. 2002. Conserv. Biol. 16(5), p.1333-1343). Thanks to the cooperation of many local fishermen it was possible to obtain relatively accurate figures although the larger purse seine fisheries vessels did not provide data. At least 922 drowned Humboldt Penguins were recorded between 1991 and 1998. Rarely more than one penguin was caught at a time by the local fishermen. Interestingly, the data showed that penguins were rarely caught in fixed gillnets anchored on the sea floor. However, the use of near-surface drift gillnets to catch e.g. cojinova resulted in penguin capture probabilities per trip of up to 58% at one site in 1994. This level of capture is clearly not sustainable. Fortunately, cojinova levels have been low in many recent years, resulting in reduced use of drift gillnets, yet the underlying problem remains. Large-scale fishing with pelagic driftnets is fortunately banned worldwide since 1991, yet this does not apply to local "artesanal" fisheries (UN Resolution 46/215).The penguin bycatch is usually eaten by the local fishermen. Whilst this is technically illegal, interfering with this practice would be counterproductive as the fishermen would then conceal their bycatch. Unfortunately, deliberate killing of penguins presumably remains a major problem (Birdlife International 2003. Web Resource: Rep. on Status and Conserv. of Humboldt Peng. & Ref. therein). Hundreds of birds have been deliberately killed every year in the past, many by guano workers or local fishermen who can access breeding sites in caves at the base of cliffs. In one case, a fishermen was observed taking 150 penguins to feed guests at a party. Penguins are also often used as bait by local lobster-fishermen. Further, the northern Chilean population has been adversely affected by illegal egg-collecting in the past. Disturbance by humans and other predators is likely to be a further driving force behind the retreat of Penguins to less accessible nesting sites at the base of cliffs and in caves. Due to the high incidence of flooding, breeding success at these sites is much lower than at cliff-top sites.Fishermen are also sometimes found keeping Humboldt Penguins as pets, thus also removing them from the breeding population. Often these were removed from nests as chicks. Pet penguins on larger ships probably account for the occasional spotting / catching of Humboldt Penguins in the northern hemisphere (Van Buren and Boersma 2007. Wilson J. Ornithol. 119(2), p.284-288). It is hypothesized that these penguins were released prior to port entry due to the fear of legal repercussions. It is unlikely that the penguins swam independently across equatorial waters. These present a barrier to penguins since they are considered too warm for penguins to cross without suffering severe heat stress.Disturbance / TourismUnfortunately, it seems that ecotourism can present a major threat to the Humboldt Penguin. Before landings were prohibited, poorly regulated visitors to the mixed colonies of Humboldt and Magellanic Penguins on the Punihuil Islands caused significant damage to the nest areas. On the most frequently visited of the two islands, 28% of dirt burrows were found to have collapsed, whereas this was only the case for 10% of those on the other island (Simeone and Schlatter 1998. Waterbirds 21(3), p.418-421). The difference was attributed largely to tourist activity together with damage by the small population of introduced goats.Even controlled ecotourism appears to be largely incompatible with Humboldt Penguins. A detailed study was performed on Damas, Choros and Chanaral Islands to determine the response of Humboldt Penguins to human presence and different forms of human approach (Ellenberg et al., 2006. Biol. Conserv. 133, p.95-106). The study revealed that the Humboldt Penguin is extremely sensitive to the presence of humans, indeed more so than any other penguin studied to date. Close direct approaches to a distance of about 2 m with a 1 min pause followed by retreat caused visual responses in the form of a series of "alternative stares" (about 30/min), where the penguin cocks its head from side to side. The heart rate (measured using a dummy egg with a microphone inside) doubled on average in response to such approaches and took on average nearly 15 minutes to gradually return to normal levels. This is unusual compared to data from other penguin species, where the heart rate rapidly declines once the human is out of sight. More crucially, an approach to 20 m stimulated a similar response and even a human passing at 150 m caused a brief about 30% increase in heart rate. This suggests that to avoid disturbance, tourists must be kept out of sight of the penguins. Indeed, the virtual complete abandonment of nesting sites on the touristically visited Damas Island, where visitors can pass along walkways through the colony, is strong direct evidence that conventional ecotourism is not compatible with Humboldt Penguins. The study also showed that Humboldt Penguins only slightly habituate to repeated human presence. It was hypothesized that exposure to human predation over the last 10000 years may have selected for particularly timid individuals. Due to the timid nature of Humboldt Penguins, it is also recommendable to sedate them during attachment of data logger devices (Luna-Jorquera et al. 1996. Marine Ornithol. 24, p.47-50).Unfortunately, it is difficult to enthuse people for endangered species if they are not able to view them. Hence, it seems that some kind of compromise may have to be made. The shy Yellow-Eyed Penguin can be viewed from a system of hides in several places, and where strict regulations are in place this has been shown to be compatible with breeding success. However, where regulation is poor, breeding sites have been abandoned under touristic pressure.DiseaseThe impact of diseases on wild populations of Humboldt Penguins has not been widely researched. However, it is evident from the many closely monitored captive populations, that they are potentially susceptible to a wide range of parasites. For example, the mosquito vector-borne West Nile Virus may lead to mortality in exposed zoo populations, to the extent that vaccination is considered (Davis et al. 2008. J. Zoo Wildlife Med. 39(4), p.582-589). Further, antibodies to Chlamydophila psittaci, Salmonella pullorum, avian adenovirus or paramyxovirus-2 were found in penguins at the Pta San Juan colony between 1992 and 1994 (Smith et al. 2008. Avian Diseases 52(1), p. 130-135). The extent to which the penguins suffered from infection could however not be established. A captive Humboldt Penguin in Japan succumbed to infection by the filarial worm Dilofilaria immitis (Sano et al. 2005. J. Parasitol. 91(5), p.1235-1237). Gastrointestinal parasites such as the helminths Tetrabothrius eudyptidis, Contracaecum pelagicum and Cardiocephaloides physalis have been found in wild populations in central and southern Chile, although their effect on the penguins is not known. Wild penguin populations are also infested with a number of ectoparasites such as fleas (e.g. Parapsyllus humboldti) and several species of ticks from the genus Ornithorodos (Hoogstraal et al. 1985. J. Parasitol. 71(5), p.635-644). Studies at the Pan de Azucar site revealed that whilst most penguins were tick-free, significant numbers of adult and nymphal ticks could be discovered around the nests, mainly under stones (Gonzales-Acuna 2008. Syst. Appl. Acarol. 13, p.120-122). The ticks were from the Ornithorodos genus. The ticks may befall humans that enter colonies and their bites cause pruritus and slowly-healing blisters. In fact, guano workers have apparently suffered severe consequences of tick bites in the past, with extensive infection around bitten areas occasionally necessitating amputations or leading to death (Clifford et al. 1980. J. Parasitol. 66, p.312-323). A role of ectoparasites in disease transmission between penguins is likely.Humboldt penguins can also develop malignant melanomas and other cancers (Rambaud et al. 2003. Vet. Record 153(7), p.217-218). However, the incidence in wild populations has not been documented and is probably negligible on a population level.PollutionPollution is obviously also a major threat to any penguin populations. Fortunately, no major oil spills have been recorded near Humboldt Penguin breeding sites in recent years. Levels of chlorinated pesticides or polychlorinated biphenyl compounds were not detectable in penguins at Pta. San Juan (Smith et al. 2008. Avian Diseases 52(1), p. 130-135). Due to the long coastline and lack of dense industrial developments in much of the breeding range, pollution with industrial chemicals will hopefully not pose a threat in the foreseeable future. |
Where To See: |
||
|